Electronic Common Technical Document - eCTD format
The Electronic Common Technical Document - eCTD format is the required format for drug product market application submissions to the FDA. The FDA is no longer accepting a paper New Drug Application - NDA, Abbreviated New Drug Application - ANDA or any annual reports, supplements, changes-being-effected - CBE and safety reports. Regulatory Operations priorities are focused on the annual submission schedule and submission due dates. ThinSpring provides outsourced eCTD submission processing services and tracks your submission end to end. You submit your eCTD submission request and upload the submission source documents securely on the ThinSpring eSubXchange portal. ThinSpring works with its partners in the United States, Canada and the European Union to create and validate the eCTD submission file. Once you review the submission, and no changes are required, you authorize submission of the file. ThinSpring tracks the submission receipts and delivers the completed package in your portal folder. All submission related documents are securely managed and tracked in one place. Email notifications and reports keep you on top of submission deadlines.
The following are examples of our eSubXchange portal screens:
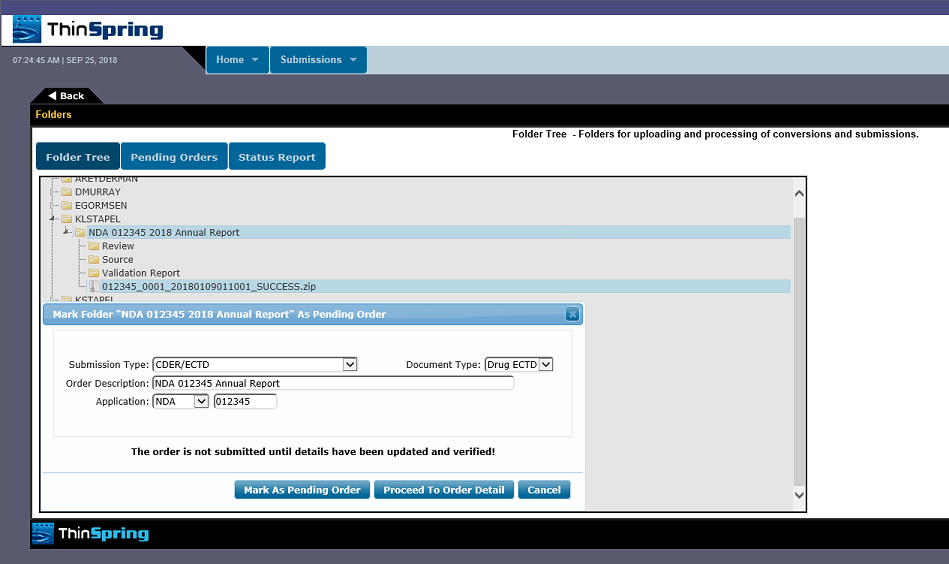
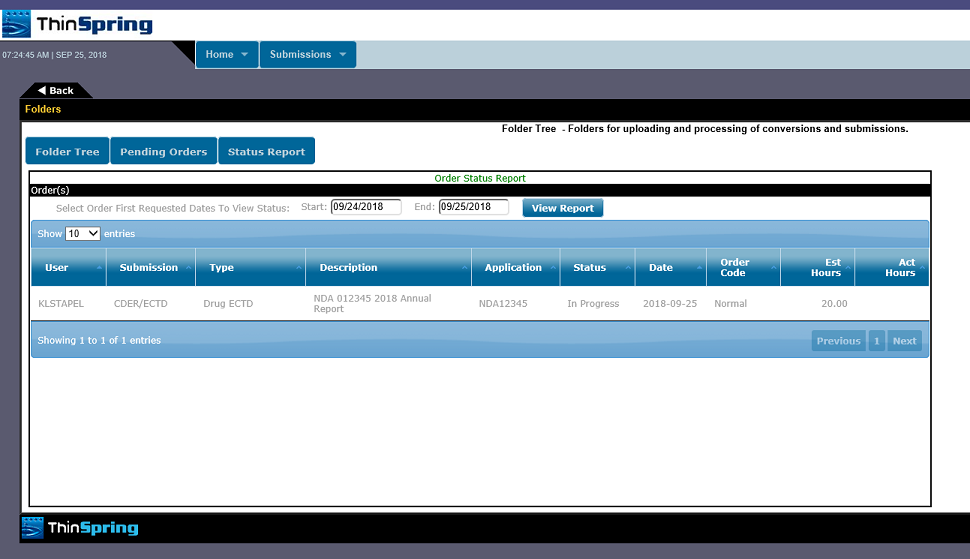
We currently support all known FDA submission types accepted by the Electronic Submission Gateway. This gateway also
services Health Canada so we can also provide HC submissions.
Click
here for a list of the current submission types

